TB, diabetes drugs to get cheaper
On May 10, 2015
The Times of India (Kolkata)
The drug price regulator has capped prices of 30 medicines, including antibiotics and those used in treatment of diabetes, tuberculosis and malaria. The move is expected to bring down prices of most of the medicines by 25-30%. However, in some cases the reduction could be as much as 50%.

Pharma cos dodging tax: CAG study
On May 8, 2015
The Times of India (Kolkata)
The Indian pharma industry has been resorting to a slew of dodgy tax avoidance practices which include claiming exemptions for illegal freebies given to doctors, research work which was not taking place, and other tricks, reveals a new report of India's audit watchdog, the CAG.
The report also takes to task the income tax department for allowing these practices causing tax losses worth crores of rupees.

Rational Drug Bulletin
Volume 24 Number 1 January 2015
Whenever a drug is prescribed for a patient, we should consider some points:
(i) Does the patient need any drug at all?
(ii) Is the drug being given to relieve symptoms, to treat the underlying condition, or to make the patient feel that something is being done for him?
(iii) Is the drug the most suitable for that patient and that condition?
(iv) Is the drug the cheapest drug of that type? If it is not, could a cheaper drug do the job as well?
(v) What side-effects may the patient suffer?
(vi) Do the possible benefits to the patient outweigh the possible risks of the drug?
(vii) How may the drug interact with the other drugs the patient is receiving?

CRACKING THE WHIP - Soon, a portal for med grievances
On March 14, 2015
The Times of India (Kolkata)
A dedicated web-based redress mechanism has been set up for consumers facing shortage of medicines or being overcharged by pharmacists.` Pharma Jan Samadhan' promises response within 48 hours of a complaint. A consumer can lodge a complaint along with the medical bill if they are overcharged for medicines or can inform the government directly in case of shortage of medicines.

Bar codes on way to check drug health
On March 14, 2015
The Times of India (Kolkata)
To ensure medicines sold in the country are genuine products, the health ministry has developed a `Track and Trace' mechanism that will enable consumers to check safety and authenticity of a drug on the internet.

Price notice to device firms
On February 23, 2015
The Times of India (Kolkata)
The government seems to be cracking the whip on medical device companies by issuing them a showcause notice for not revealing their prices.
Sources said the government was keen to monitor prices of medical devices such as exorbitantly-priced cardiac stents and implants, so as to prevent patients being overcharged. It has set a deadline of two weeks for the details to be submitted.

Med bills must show price cap
On February 23, 2015
The Times of India (Kolkata)
The government, in an attempt to empower consumers and save them from paying more for medicines, is set to make it mandatory for chemists to mention in the bill whether they are selling a price-controlled product or not.

Big boost: New drug raises hope for an HIV vaccine
On February 20, 2015
The Times of India (Kolkata)
Scientists have developed a novel drug candidate that may lead to a potent and universally effective HIV vaccine. Researchers found that the new drug candidate blocks every strain of HIV-1, HIV-2 and SIV (simian immunodeficiency virus) that has been isolated from humans or rhesus macaques, including the hardest-to-stop variants. It also protects against muchhigher doses of virus than occur in most human transmission and does so for at least eight months after injection.

Patients spend Rs 2,500cr on stents every year in India
On February 20, 2015
The Times of India (Kolkata)
Following Norms Would Have Saved Rs 1,500cr
Patients spend about Rs 2,500 crore on cardiac stents alone every year in India
even by conservative estimates, the bulk of it being paid from their own pockets.
This does not include the cost of blood tests, angiography, procedures, charges
for hospital stay, doctor's charges and so on.
An estimated four lakh stents were implanted in India in 2014. Of this, over 85%
were drug eluting stents (DES), for which most patients pay anything between Rs
55,000 and Rs 80,000. Annually, the stent market is estimated to grow by about 15%,
with the growing incidence of cardiovascular diseases. Hence, the market is expected
to grow steadily.

Many Indian drug combos harmful: Study by Rupali Mukherjee
On February 10, 2015
The Times of India (Kolkata)
A study in respected medical journal Lancet has ripped apart India's drug regulatory
system and the domestic pharma industry, saying the Indian Drug Act makes it possible
for harmful fixed dose combinations (FDCs) to evade both approval and price controls.

`80-90% crucial drugs imported from China'
On January 29, 2015
The Times of India (Kolkata)
If Prime Minister Narendra Modi's `Make in India' mantra has to be implemented in
some sector first, it has to be in the pharma industry.

Drugs sold online sans prescription
On January 29, 2015
The Times of India (Kolkata)
Despite Risk, Biz Thrives In Absence of Policing
A prescription for disaster is being written on the internet, in India, as unauthorized
buyers log on to get drugs including stimulants and anti-depressants that should
be sold only with a physician's prescription.
Inquiries by TOI found that plenty of websites take orders and deliver the drugs
at the buyer's doorstep through courier. A call to a popular online supplier of
drugs in New Delhi, which operates on the web under `USA medications without prescriptions'
tag said they sell all kinds of medicines from Xanax (anti-anxiety drug) to Ephedrine
(a stimu lant) and Viagra.

44% advised surgeries needlessly, finds survey `Even Docs Seek 2nd Opinion If Told
To Go Under Knife'
On January 04, 2015
The Times of India (Delhi)
Is surgery necessary? A medical second opinion services centre has found an uncomfortable
answer to this question that traumatizes every family when a dear one is advised
surgery. Almost 44% of the 12,500 patients for whom surgery was recommended were
advised against it by their second opinion consultants.

Drug price watchdog in states?
On January 04, 2015
The Times of India (Kolkata)
The government plans to spread its regulatory wings to keep medicine prices under
stringent check in every nook and corner of the country and save consumers from
paying more.
It is working to set up drug price monitoring cells across the country to keep a
close watch on real-time price movements, the maximum retail price of medicines
and their availability, a senior official said.

Govt to Priorities Drugs, States to Revive Jan Aushadhi Pharmacies
On January 02, 2015
The Economic Times (Kolkata)
Govt may trim distribution list for such outlets to 40-50 mass consumption drugs
To infuse a fresh lease of life into the struggling Jan Aushadhi chain of pharmacy
outlets, set up to make affordable generic drugs available to people, the government
plans to focus on select drugs and states, spruce up supply chain and encourage
doctors to use generic names of drugs.

Competition, Delay in Drug Approvals May Take a Toll on Pharma Cos' Health
On January 02, 2015
The Economic Times (Kolkata) - Kiran Somvanshi
With global macro-economic scenario turning volatile, Indian players may have to
strategise harder to stay ahead
For investors, the export-oriented, defensive pharma sector produced high returns
(of over 45%) during 2014. The year was indeed an important one for the sector -the
Sun-Ranbaxy Deal, recovery in domestic market in the aftermath of price control,
record sales in the US -all resulting in its out-performance on the bourse. With
the global macro-economic scenario turning volatile, there are signs that the going
may not be as good in 2015.

Infections resistant to 'last antibiotic' emerge in India
On December 29, 2014
The Times of India (Kolkata)
It is the beginning of the end. Hospitals in India are now recording cases of infections
resistant to colistin, the last antibiotic available in the world. It was brought
back from a 40-year exile in 2005 to treat increasing number of infections resistant
to other high-end antibiotics. For now, colistin is the only cannon left in the
medical armoury to treat bacterial infections, mainly those acquired in the hospital
that no drug can treat. The number of such cases is rare, but worrisome, say doctors.

MED DATA BOOST - Report drug side-effects on govt toll-free helpline
On December 19, 2014
The Times of India (Kolkata)
Now, consumers can call to directly report adverse reactions or their bad experiences
from any medicine.
The health ministry has launched a toll-free number where people can call and report
the side-effects and problems faced by them along with details of the medicine suspected
to have caused the adverse reaction.

Gearing Up to Meet Healthcare Needs
On November 19, 2014
The Economic Times (Kolkata)
The market for chronic illness medicines has grown at a much faster rate than that
for other ailments The last five years has seen the highest volume growth in anti-diabetic
medicines followed by those for treating semi-chronic ailments in urology (urinary-related)
and dermatology (skin care).

Ranbaxy Sues US FDA for Revoking Nod to Generics
On November 19, 2014
The Economic Times (Kolkata)
Says FDA has overstepped jurisdiction by revoking approvals given years back Ranbaxy
Laboratories has sued the US Food and Drug Administration (FDA) in an American court
for revoking approvals the company had to market generic versions of patented drugs
exclusively for six months in the US market -Astra Zeneca's anti-ulcer pill Nexium
and Roche's anti-viral Valcyte. Ranbaxy's marketing opportunity from this has been
estimated by analysts at upwards of $200 million. Of this, they estimated revenue
from generic Nexium to top $160 million.

Heart disease hits Indians early, diabetes, high BP make it worse - The Times of
India (Kolkata)
On September 30, 2014
Malathy Iyer, Mumbai
In the Indian pool of heart patients, almost every second patient has high blood
pressure, every fourth has diabetes and every fifth had plaque deposits in his her
arteries.

Stop prescribing drugs for fever, cold, medical body will tell docs - The Times
of India (Kolkata)
On September 27, 2014
DurgeshNandan Jha, New Delhi:
`Overuse Of Meds A Major Health Risk' Faced with the scary prospect of losing lives
to simple infections in the future, India is finally waking up to the dangers of
reckless antibiotic use. The Indian Medical Association, a pan-India voluntary organization
of doctors, will on Sunday launch a nationwide awareness programme on overuse of
the life-savers, a practice that has led to the emergence of drug-resistant organisms.
The IMA will also ask fellow practitioners to avoid unneces sary prescriptions such
as recommending antibiotics for patients with fever and cold, which are generally
caused by viral infections.

India withdraws regulator's power to cap non-essential drug prices
On September 23, 2014
By Aditya Kalra and Zeba Siddiqui
NEW DELHI/MUMBAI (Reuters) - India's drug pricing authority said the government
has withdrawn its power to set prices of non-essential medicines, but price caps
on over 100 non-essential drugs that drew the industry's ire in July will remain.

Indian Firms Make Inferior Drugs for Poor Nations: Report
On September 16, 2014
India-made drugs sold in Africa are inferior and of poorer quality when compared
with those sold in India and other middle income countries, alleges a new paper
by an US think tank American Enterprise Institute (AEI), the findings of which are
scheduled to be announced in a briefing at the Capitol Hill on Wednesday.

The Most Expensive Drugs Sold in India
On September 16, 2014
In 2006, US drug maker Gilead Sciences Inc introduced the concept of voluntary licences
in India, where it gave permission to some Indian companies to manufacture and sell
its anti-HIV drug Viread. Now, Gilead has applied the same strategy for Sovaldi,
considered to be a path-breaking treatment to cure Hepatitis C infection.

Generic drugs skip quality control test, govt apathetic
On September 16, 2014
While the Indian government is pushing generic drugs as they are cheaper and, therefore,
more affordable, there seems to be inadequate attention on ensuring that the quality
protocol of these drugs is properly observed.

Hepatitis C drug to cost Rs 49L less than in US
On September 16, 2014
Sofosbuvir, the wonder medicine for Hepatitis that costs $84,000 or Rs 50.4 lakh
for a 24-week treatment regimen in the US, will soon be available in India for about
$1,800 or roughly Rs 1.1 lakh for the same regimen.The patent holder, pharma major
Gilead, announced on Monday that it would sell the drug at this price in India and
also give voluntary licences to seven Indian pharma companies to produce it.

Standard Treatment Guidelines, Organized by CDMU in collaboration with SIGN & FSI,
Patna
On September 10, 2014
On September 10, 2014 the 2nd edition of Standard Treatment Guidelines [STG] prepared
by CDMU in collaboration with Social Initiatives for Growth and Networking and Forum
for Social Initiative, Patna was launched.

2nd edition of Standard Treatment Guidelines lauched
On July 21, 2014
On July 21, 2014 the 2nd edition of Standard Treatment Guidelines [STG] prepared
by CDMU in collaboration with Social Initiatives for Growth and Networking was launched.

Key Diabetes, Cardiac Drugs to get cheaper
Rupali Mukherjee, July 14, 2014
Copying with the inflation demon will become a tad easier for the burdened consumer.
In a move that has surprised and shaken the industry, prices of widely used expensive
antidiabetic and cardiac medicines will reduce over the next few weeks by as much
as 35% with the drug pricing regulator, National Pharmaceutical Pricing Authority
(NPPA), deciding to bring them under price control.

Use of antibiotics in India rises 62% in 10 yrs: Study
Kounteya Sinha, July 14, 2014
BRICS Nations Lead Spurt Worldwide From 2000-10
India has emerged as the world's largest consumer of antibiotics, with a 62% increase
in use over the past decade.
Global Trends in Antibiotics Consumtion, 2000-2010, a study by scientists from Princeton
University, has found that worldwide antibiotic use has risen by 36% over those
10 years, with five countries - Brazil, Russia, India, China and South Africa (BRICS)
responsible for more than threequarters of that surge.

Pharma co prices breast cancer drug in tiers
Rupali Mukherjee TNN, November 6, 2013
Mumbai:The $7-billion Japanese pharmaceutical company Eisai, which set up operations
in India nearly eight years ago, is extending its affordable pricing strategy by
introducing a critical breast cancer drug through a tiered model. The exorbitantlypriced
drug Halaven, which costs around Rs 4.8 lakh per four cycles, prescribed as a third-line
treatment for metastatic breast cancer, will be offered to those from lower socio-economic
classes free of cost. This is in addition to the two other medicines already sold
under the affordable pricing strategy at one-tenth of the global price, says the
company’s Asia deputy president Yuji Matsue.
Innovative drugs, anti-Alzheimer’s drug Aricep and gastro-intestinal drug Parit,
are already sold under an affordable pricing strategy here.

Eisai joins other drug biggies like GSK and Sanofi, which have advocated a differential
pricing model that includes offering exorbitantly-priced life-saving medicines at
a fraction of the cost in developing countries like India. A major challenge for
drug companies in India is reaching out to patients who need critical healthcare
and making treatment costs affordable. Japan’s fifth-largest drug company plans
to focus on its two major pillars — treatment for central nervous treatment (CNS)
and oncology in India, and has taken the organic route for growth, which is in line
with its traditional philosophy, said Matsue.
Under the tiered-pricing model, the cost burden to patients is differentiated according
to their income level, ranging from full payment by the patient to total reimbursement
by the company.
The prevalence of breast cancer in India is estimated to be around 3.4 lakh cases,
while the mortality rate in breast cancer is very high, as few patients are diagnosed
and treated. It has recently introduced a higher dose, 23 mg, of its blockbuster
drug Aricep for anti-Alzheimer’s treatment, which is available at one-tenth of the
global price here, Eisai Pharma India MD K Shivkumar said. In fact, Aricept (brand
name in US) with sales of $3 billion, lost patent protection in 2010, while Eisai’s
largest selling drug globally now is Parit (Rabeprazole) with sales of around $1.1
bn.
Eisai’s presence in India goes back to 1999 when it outlicensed a key neuropathy
drug, Methycobal, to Wockhardt and followed it up with an alliance with GlaxoSmith-Kline
for its gastro-intestinal blockbuster Parit. It also has a tie-up with Unichem for
a muscle-relaxant pain reliever. These agreements will continue to be in place,
Matsue said.
The Japanese firm plans to appoint a field force of 15 to promote the life-saving
cancer drug to cover about 400-odd oncologists across India.
Kolkata, Howrah hit hard by diabetes
TIMES NEWS NETWORK, November 5, 2013
Kolkata: Urban areas of Bengal — Kolkata and Howrah in particular — have the highest
prevalence of diabetes in the state but awareness on the disease nicknamed ‘silent
killer’ is still low.
Diabetes is a metabolic disease in which a person has high blood sugar, either because
the pancreas does not produce enough insulin, or because cells do not respond to
the insulin that is produced.
While 3.5%-5.7% of Bengal’s population is diabetic, the figure shoots up for Kolkata
(12%) and Howrah (13.2%). “There are mainly three districts — Kolkata, Howrah and
Burdwan (8.7%) — where the rate of diabetes is higher than other areas because of
mass urbanization coupled with stress-related problems. Diabetes can be controlled
if treated at an early stage but most people come to us very late, sometimes even
10 years late, so the chances of getting cured become remote,” said Subhankar Chowdhury,
secretary of Research Society for the Study of Diabetes in India (RSSDI).

“We will organize a Madumeha Mela (Diabetes Fair) at Netaji Indoor stadium on November
13, which will be inaugurated by minister of state for health Chandrima Bhattacharya.
Here, people can get their blood sugar checked, measure their body fat percentage,
estimate the possibility of heart attack and stroke and have their eyes examined
for diabetic retinopathy,” Chowdhury said.
The organization will illuminate MP Birla Planetarium in blue light — the colour
for diabetes. This will be launched by sports minister Madan Mitra. “On November
14 — World Diabetes Day — diabetic patients, schoolchildren, doctors, healthcare
professionals and eminent personalities will walk from Victoria Memorial to Netaji
indoor stadium at 9am after being flagged off by presidentelect of the International
Diabetes Federation, Shaukat M Sadikot,” Chowdhury said.
City doctors offer answer to random use of antibiotics
Prithvijit Mitra TNN, November 5, 2013
Kolkata: Doctors in the city will soon be able to refer to a website and choose
the best possible antibiotic for a particular bacteria. An antibiotic protocol is
on the verge of being put together by a 15-member “Task Force for Antibiotic Stewardship
Kolkata” that comprises doctors from five city hospitals.
The task force is ready with a software — titled “The Life” — that can analyze data
on bacterial prevalence and drugs used to combat them and offer the best possible
antibiotic solution for individual patients. To be uploaded in January, the software
will be free and accessible to all.
A huge volume of data on bacteria, drugs used to combat them and the results has
been gathered by the task force from five hospitals — Ruby General, Fortis, Medica
Superspecialty, KPC Medical College and Saroj Gupta Memorial Cancer Research Institute
(SGMCRI), Thaukurpukur. “We will analyze the data that will give us an idea about
the kind of bacteria that’s prevalent in various parts of the city and its outskirts.
It’ll also throw light on the kind of antibiotics that are proving to be effective
in terminating the bacteria. So far, we have not had any information on these. Once
we have that, it will be easier to draw up an antibiotic protocol that’s necessary
to prevent antibiotic resistance,” said Dayanath Mishra, director of DM Hospital
and a member of the task force.

Around 65% of those admitted to ICUs of hospitals in Kolkata are believed to be
resistant to multiple antibiotics, rendering them vulnerable to infections. Around
60% of them don’t respond to treatment. Nearly 10% of patients show multiple-drug
resistance. Irrational use of antibiotics is believed to be the reason. Experts
point at over-the-counter purchase of antibiotics and ignorance about drug resistance
among medical practitioners as the reasons. Though it is widely known and often
debated, little has been done so far to stop the killer practice.
“The only way to fight resistance is to stop the random use of antibiotics. Bacteria
change character and turn resistant. The more patients are exposed to antibiotics,
the less effective they will be. We need to set aside some to treat extreme cases.
But that’s not happening. Once we have a protocol, resistance can be dealt with
effectively,” said Arnab Gupta, director of SGMCRI.
The protocol will also cut down the presumptive treatment period, explained doctors.
“Once the blood culture report is available in two-three days, we can narrow down
to just one antibiotic. The rest can be safely discontinued. Once we have the protocol,
we can be reasonably sure about the effectiveness of the antibiotic being prescribed,”
said Mishra. “The software will let doctors make a more informed guess while prescribing
antibiotics before the bacteria is detected. Once the reports are available, both
the antibiotic and the dosage can be altered if necessary. But it will cut out the
blind and random prescription of antibiotics,” said Gupta. He added that major antibiotics
like Piperacillin-Tazobactum and Sulleractum Pefaferezone have turned ineffective
due to random use.
But the protocol can be effective only if hospitals across the city adopt it, pointed
out experts. “Only a handful of private hospitals can’t make it work. We need a
coordinated effort and a uniform strategy. Whichever hospital or doctor a patient
goes to, the antibiotics taken by him need to be scanned, along with the bacterial
attacks he has suffered in the past. This will be easy once the software is ready.
It has to be borne in mind that individuals react differently to different antibiotics.
While a first-generation antibiotic might work for some, another might need a combination
of multiple antibiotics to counter the same bacteria,” explained Mishra.
Plan panel aims at cheaper drugs for all
Times News Network, September 26, 2013
Kolkata: The Planning Commission is embarking on a project which would develop a
system to provide affordable, acceptable and accessible medicine for every Indian,
said Arun Maira, member, Planning Commission, Government of India.
“We will be starting the process of identifying and inviting key stakeholders who
would facilitate the project,” said Maira, on the sidelines of a seminar organized
by ICRIER-KAS-FICCI. “We are hoping that the roadmap of the project would be formulated
by the middle of next calendar year,” he added.
Maira mentioned they have a partner, who will be identifying stake holders and mapping
the systems. “A report on this will be presented to the commission by October,“
said Maira.

He said that every one, including international pharmaceutical companies, have agreed
for developing this system. “The conversion of contention into collaboration is
very essential,” he added.
“There are many grave issues in the country concerning generic and patented drugs
along with price and quality regulations. Since the last two years, there has been
no clear strategy. Its just muddling along in terms of healthcare,” mentioned Maira.
According to him, all parts of the system should be brought together to make or
shape a strategy.
India is the biggest foreign supplier of medicines to the US and is home to almost
200 FDA approved drug manufacturing facilities, including many run by multinational
players. Pharmaceutical exports from India to the US rose nearly 32% last year,
to $4.2 billion. India accounts for nearly 40% of generic drugs and over-the-counter
products and 10% of finished dosages used in the US.
The share of other major generic drug-manufacturing countries, such as Japan, Israel
and China, is much lower, analysts suggest. Mamata is right to seek more power for
states, says Maira
Supply essential drugs: Govt to cos, stockists
Rupali Mukherjee TNN, September 24, 2013
Mumbai: Perturbed by reports of shortage of essential medicines on retail shelves
after the implementation of new pharma policy, the government has sent out a sharp
message to drug companies and trade channels to ensure their availability across
the country. Supplies of widely used medicines such as pain relievers paracetamol
and diclofenac, treatment for worms albendazole and those used in chronic ailments
like cholesterol-lowering drug atorvastatin, diabetes drug metformin and blood pressure
drug enalapril have been affected.
The drug pricing regulator, NPPA (National Pharmaceutical Pricing Authority), in
a strongly worded communication to pharma companies and distribution channels, has
warned that the Essential Commodities Act may be invoked against those who disrupt
the supply and distribution of essential medicines.

The supply of certain crucial medicines used in tuberculosis and leprosy treatment
and injections oxytocin, metylergometrine and amikacin used in surgeries and deliveries
have also been disrupted over the last couple of months, industry sources told TOI
(see chart).
The NPPA, citing the Drug Price Control Order (DPCO) 2013, has said in the letter
that no drug formulation can be sold to a consumer at a price exceeding the one
notified by the government or the one printed on the medicine pack. The government
notified in July ceiling prices of certain medicines which are part of the National
List of Essential Medicines. Citing Para 28 of the DPCO 2013, the NPPA communication
said that no manufacturer can refuse to sell a drug to a distributor while no distributor
can withhold the sale of a drug to a consumer planning to purchase the medicine.
Representation from the industry association and reports received indicate that
there is a disruption in supplies of key medicines due to withholding sale of certain
medicines by stockists and retailers protesting against the trade margins, the letter
said.
Since the implementation of the new pharma policy, a tussle has been on between
pharma companies and trade channels over margins, with stockists reducing their
orders leading to scarcity of widely prescribed medication like painkillers, anti-infectives,
cardiac drugs and antibiotics. Supplies of essential medicines have been particularly
disrupted in Gujarat, Karnataka, Tamil Nadu, West Bengal and Jharkhand. Major companies
like Cipla, Mankind Pharma, FDC and Torrent have given in to the demand of higher
trade margins, hoping to end the stalemate between the industry and chemists. Drug
major Cipla and Mankind Pharma increased trade margins – 10% to stockists and 20%
to retailers – on the price-controlled basket of drugs, as against the earlier offered
8% and 16%, respectively. Others like Torrent and Eris Lifesciences are doling out
a 5% “special discount” on these medicines. The DPCO 2013 stipulates a trade margin
of 16% to retailers while to wholesalers the industry offers a trade margin of 8%
on price-controlled drugs, continuing with the earlier practice. For medicines out
of price control, trade channels continue to get margins of 10% and 20% as earlier.
Accreditation mandatory for clinical trials
Shobha John TNN, September 19, 2013
New Delhi: In an attempt to bring about reforms in clinical trials, a six-member
expert panel constituted by the ministry of health and family welfare has said that
in future, these trials can only be carried out in accredited centres where the
principal investigator is also accredited.
These recommendations, already on the website of the Central Drugs Standard Control
Organization (CDSCO), are part of the Professor Ranjit Roy Chaudhury Expert Committee
on New Drugs and Clinical Trials Approvals and they attempt to weed out fly-by-night
operators who collude with drug companies and doctors to approve drugs whose trials
have never taken place.
“While some of the recommendations can be implemented within two months after consultations,
others will require an amendment of rules,” says a health ministry official.

Dr Roy Chaudhury, adviser to the Department of Health and Family Welfare, says the
lack of regulation in clinical trials has seen India lose out to China, Malaysia
and Singapore.
Another recommendation was to set up a Central Accreditation Council to oversee
these accreditations. Selection of experts to review new drug applications will
be randomly made from a roster of experts, prepared after a nationwide search. A
roster of accredited institutes for clinical trials will be made and pharmaceutical
houses can choose from these.
To reduce bureaucratic tangles, the present 12 drug advisory committees will be
replaced by one Technical Review Committee. The CDSCO should give a written assurance
to a pharmaceutical company seeking approval for a trial that a decision will be
given within three months if all papers are in order.
For the drug trial itself, consent from participants is mandatory. For special groups
which can’t protect their interests, consent will have to be taken from the guardian
in the presence of an independent person who will also have to sign the consent
document. Audiovisual recording of this is mandatory.
46 drugs to be sold only on prescription
From March ’14, Chemists Will Have To Keep Record Of Prescriber, Buyer
Times News Network, September 18, 2013

New Delhi: Habit-forming antibiotics, anti-TB and other such drugs like sleeping
pills will not be freely available at chemists from March 1, 2014, following a government
notification that regulates the use of 46 such medicines.
These drugs won’t be available over-the-counter (OTC), and will be sold only through
a doctor’s prescription. Chemists will have to record the names and addresses of
prescriber and buyer of all such drugs in a separate register. As per the notification,
such records will have to be maintained for three years and they will be open for
inspection by the regulatory authority, the Drug and Controller General of India
(DCGI).

Such drug formulations in Schedule H1 will also carry a symbol “Rx” printed in red
colour on the top left-hand corner of the label.
The notification also says such drugs will carry a warning that they are habit-forming
and can be dangerous and that they can be sold only through prescription by a registered
medical practitioner.
Such a warning should be labelled in a box with red border, cautioning a buyer,
“it is dangerous to take this preparation except in accordance with the medical
advice” and “not to be sold by retail without the prescription of a registered medical
practitioner.”
Some such drugs are anti-TB, while some are normal medicines used for sleeplessness.
The notification said preparations containing the above drug substances and their
salts excluding those intended for topical or external use, except ophthalmic and
ear or nose preparations, containing above substances are also covered by the new
Schedule H1.
These have been notified after the approval of the Drug Technical Advisory Board
(DTAB) which is chaired by the Director General of Health Services (DGHS).
Crowdsourcing drive for TB, malaria drugs
Himanshi Dhawan TNN, September 18, 2013

New Delhi: Giving crowd sourcing a whole new meaning, scientists at the Council
for Scientific and Industrial Research (CSIR) have initiated a country-wide venture
to build a chemical library with diverse compounds that will successfully drive
drug discovery programmes, particularly for neglected diseases like tuberculosis
and malaria.
CSIR had launched the Open Source Drug Discovery (OSDD) in 2008 with the objective
of discovering drugs for neglected diseases like TB, malaria and others through
open innovation and sharing of research that has been lauded across the globe.
Building on the current programme is OSDD Chemistry outreach programme (OSDDChem).

Initiated recently, students are trained in synthetic chemistry and the compounds
synthesized in the universities, institutes and colleges in OSDDChem centres are
submitted to the OSDDChem database and sent to CSIR-CDRI.
These molecules are then taken up for screening at CSIR-CDRI for anti-TB and anti-malarial
activity.
According to scientists, lack of chemically diverse compounds has been recognized
as a crucial factor for the poor success rates of anti-infective drug discovery
and development in the past years.
So far, there are 34 academic institutions, including IITs in Delhi, Kharagpur,
Madras and Bombay, Universities of Calicut, Kerala, Jammu, Pondicherry and Delhi,
are participating in the project.
Not only has OSDDChem succeeded in developing a national online repository of small
drug-like molecules, it is now venturing into building chemical libraries with diverse
compounds for driving successful drug discovery programs.
“This aligns with the OSDD policy of ‘no molecule will be left behind’ for screening
against neglected diseases and the assurance that the molecules submitted to OSDD
will be taken up for screening against neglected tropical infections like TB and
malaria,’’ a scientist said.
OSDD project director and scientist Zakir Thomas points out that pharmaceutical
companies are keen in investing in therapeutic areas with large revenues neglecting
research and development in tropical infections like TB, malaria and leishmaniasis.
The global market for TB is $300 million, he says, and hence not large enough for
pharmaceutical firms, especially multinationals, to invest in.
State staring at shortage of drugs
Pushpa Narayan TNN, September 01, 2013
Three major drug manufacturing associations have warned that at least four states
— Bengal, Tamil Nadu, Karnataka and Gujarat —will soon face a huge shortage of essential
drugs as many distributors and retailers looking for more profit aren’t stocking
them.
More than a month ago, the Centre revised the National List of Essential Medicines
thus bringing down the prices of more than 300 essential drugs. The price of paracetamol,
for instance, was reduced to less than a rupee. Prices of diabetes medicines, glibenclamide
and metformin, and blood thinner medicines for people with hypertension were also
slashed.
However, the revision hit profits for distributors and retailers, many of whom refused
to lift stocks at the revised rates. Pharma body wants state to intervene to avoid
shortage

A profit margin of 10% for the distributor and 20% for the retailer on many medicines
has come down to 8% and 16% respectively. “Since then, there has been an unofficial
boycott. Some have refused to buy and stock these medicines at the revised rates.
Many pharmaceutical companies have seen a drastic reduction in orders. If the situation
continues, we may soon have a serious drug shortage,” said Mumbai-based D G Shah,
secretary general, Indian Pharma Alliance, an industry body of leading domestic
pharmaceutical firms.
A week ago, the Indian Pharma Alliance along with Indian Drug Manufacturers Association
and Organisation of Pharma Producers of India sent a letter to four state directors
of drug control (Bengal, TN, Gujarat, and Karnataka) asking them to intervene. In
the letter, they said the apprehension of potential shortage was based on the lack
of orders for essential medicines. It urged the state to check the availability
of essential medicines in the state and take necessary steps to ensure uninterrupted
supply to customers.
“A similar problem was faced in Maharashtra and Kerala but timely action by the
state FDAs ensured the resumption of purchases and prevented shortages of essential
medicines. The governments there threatened to cancel licences of traders who did
not stock the medicines. Druggists and retailers in places like Chennai are exhausting
their stock,” said S V Veeramani, vicepresident, All India Drug manufacturers Association.
Pharma traders’ associations refused to comment on record. But they said they were
finding it difficult to handle the losses with new prices. They said there has been
an increase in wages, transportation costs and electricity costs with a drop in
turnover in the last year.
Panel raps govt over clinical trials, lapses
Rupali Mukherjee TNN, August 31, 2013
Mumbai: In a further indication of the rot in the country’s healthcare system, a
parliamentary panel has rapped the government for gross irregularities in drug trials,
under-reporting and lapses in monitoring serious adverse events and lethargy in
safeguarding health, in studies on cervical cancer prevention vaccine by a US-based
non-governmental agency. Charging the government for inaction, the parliamentary
committee on health says in a report that the issue has been diluted with no accountability
fixed on erring officials for serious violations committed in the studies which
led to the death of hapless tribal children three years back.
Raising concern on the manner in which the US NGO, PATH (Programme for Appropriate
Technology in Health) set up office in the country, the panel says it conducted
clinical trials for HPV vaccines under the garb of “observational project” by violating
all guidelines.
The trials were suspended following deaths of five girls in Andhra Pradesh, and
two deaths in Gujarat in 2009-2010 after being administered the HPV vaccines. The
vaccines were provided by two pharmaceutical companies — Merck and GlaxoSmithKline
— through PATH, during studies carried out in collaboration with government agency,
Indian Council of Medical Research and the states. The project was reportedly funded
by Bill and Melinda Gates Foundation.

The sole aim of PATH, the 72nd report on Alleged irregularities in the conduct of
studies using Human Papilloma Virus (HPV) vaccine says, was to “promote the commercial
interests” of the manufacturers, who would have benefitted if it was successful
in getting the HPV vaccine included in the government-run Universal Immunization
Programme.
The report submitted in the Parliament on Friday comes at a time when the pharma
industry has been complaining of inordinate government delays in approvals of drug
trials involving human subjects.
Terming it “a serious breach of trust by any entity”, and a “violation of human
rights of these girl children who were mostly unaware of the implications”, the
panel has asked the government to report the violations to international bodies
like World Health Organisation and UNICEF. It has also asked the ministry of health
to take up the matter through the ministry of external affairs with the US government
so as to ensure appropriate action is taken against PATH.
The report has pulled up the drugs controller general as well as Indian Council
of Medical Research. It says DCGI played a “questionable role”, and “remained a
silent spectator, even when its own regulations were being violated”, while “approvals
of clinical trials, marketing approvals and import licenses appear to be irregular”.
Though the issue was reported after the deaths in 2010, this report details the
role of PATH, involvement of regulatory agencies like ICMR and DCGI, lapses in drug
side-effects, conflict of interest and the various loop-holes in the system.
The panel says that ICMR instead of ensuring ethical standards in research studies,
apparently acted at the behest of PATH in promoting the interests of HPV manufacturers,
and that it should have taken the National Technical Advisory Group on Immunization
(NTAGI), on board. The safety, efficacy and introduction of vaccines is handled
by NTAGI.
Lack of clarity hits drug pricing plan
Rupali Mukherjee TNN, July 30, 2013
Mumbai: The watershed drug pricing policy that was to have kicked off on July 29
after a decade of intense debate and planning, is now threatened by a variety of
reasons including court cases and bureaucratic lethargy. Confusion in the pharma
industry persists along with a fear of shortage of essential drugs, with companies
moving court to delay implementation of the new drug price control order (DPCO)
and industry organizations seeking more time to comply.
The chaos has led to companies and trade channels being unclear about complying
with the 45-day deadline of introducing new packs with the revised price. As a result,
retail shelves will still have old stocks with no change in prices, while companies
and trade grapple with the logistics mess involved in recalling and supplying medicines.
The 45-day deadline of the first list of 151-odd medicines, including antiinfectives,
anti-diabetics and antibiotics whose prices were to be revised as part of the DPCO
2013, ended on Monday.

Packs of these medicines with the revised prices should be on the shelves from Monday,
but experts say that the entire exercise has been botched up. Sources from trade
channels told TOI that only 10-15% of the 151 medicines, with packs reflecting lower
MRPs (maximum retail prices), had reached the market till now.
A shortage of premium priced medicines looms large as also of those for critical
diseases like TB, chronic ailments like diabetes and hypertension, and widely used
antibiotics, they added. Ranjit Shahani, president of industry body OPPI, said:
“We have sought an extension of 45 days to comply with the DPCO as logistics is
a challenge. Supplies with the revised packs would also be delayed because of the
heavy rains in the country.”
The industry is still awaiting a clarification from the finance ministry which will
make “stickering’’ excise duty free. This will basically mean that companies will
not pay excise duty once again on the packs which they send out of their factories.
Also, on Monday, Sun Pharma and industry organization CIPI are understood to have
filed cases in the Delhi high court against the pricing policy, joining others like
Cipla and Alembic who have earlier voiced concern. Sun Pharma declined to offer
comments on the details of the case. Cipla has raised concerns over the government’s
directive to replace stocks in the market with those carrying reduced prices.
The exercise of recalling and supplying medicines is tedious as the formulations
run into thousands. Recalled stocks can get spoiled in transit, while there are
chances that the stickers (with revised prices) may come off from the packs, say
experts
Health min ups ante against patents
Pushes For Use Of Rare Clause To Revoke Patent Of Breast Cancer Drug
TNN, July 23, 2013
New Delhi: The health ministry has asked for a cancellation of patent to Trastuzumab
— a medicine which treats a form of breast cancer — using a rarest of the rare provision
in the Indian Patents Act. The move comes after the department of industrial policy
and promotion (DIPP) turned down a plea for a compulsory licence, or suspension
of the patent, to make the medicine more affordable.
Industry department has also opposed the move to cancel the patent of Swiss drug
major Roche, arguing that it was already facing post-grant opposition in the Patents
Office. The health ministry had suggested that the government use powers under section
66 of the Indian Patents Act to revoke the patent in public interest. The government
has used the provision to revoke patents only twice. In 1994, it cancelled a patent
given to a US firm for developing cotton cells by tissue culture while last year
it used the power for a medicine made of jamun, lavangpatti and chandan meant to
treat diabetes.

Besides, the government does not want to use power available with it to meet all
demands, given the international scrutiny such actions face. Already, the use of
compulsory licence provisions for a renal cancer medicine, for which Bayer has a
patent, to bring down the cost has put the spotlight on Indian policymakers. Similarly,
developed countries, led by the US, have been critical of other provisions in the
Indian Patents Act that allow the patent controller not grant exclusive rights in
case of tweaking or “evergreening”.
In a landmark ruling involving Novartis’s anti-cancer drug Glivec, the Supreme Court
upheld the validity of the provisions to check frivolous patents. The Indian government
has argued that these flexibilities are provided under the World Trade Organisation’s
Agreement on Trade Related Aspects of Intellectual Property Rights (TRIPS).
Campaign for Affordable Trastuzumab, an association of breast cancer survivors and
civil society groups, had written to the government seeking use of compulsory licence
provisions to lower the cost of the medicine from around Rs 8 lakh for a full course
of 12 injections. There have been demands that the drug should be offered free in
government hospitals.
Under TRIPS, a government can issue a compulsory licence, which allows a company
to manufacture or sell a patented drug to meet national health emergency.
US pharma co sues Indian drug makers
Mumbai: US-based biopharma company UCB, along with certain other companies, has
filed separate cases in the US against as many as 15 drug makers — including domestic
companies Ranbaxy, Aurobindo, Zydus, Sun Pharma, Glenmark and Alembic Pharmaceuticals
— for infringing its patented drug Vimpat used in the treatment of epilepsy. According
to a petition filed by UCB Inc, UCB Pharma GMBH and others, the patent on its drug
Lacosamide tablets 50 mg, 100 mg, 150 mg and 200 mg would expire on March 17, 2022.
The patent infringement petition was filed in the US district court of Delaware.
For the 12 months ended March 31, 2013, Vimpat had US sales of approximately $338
million, according to IMS Health. The drug is approved as an adjunctive therapy
to treat partial-onset seizures of people diagnosed with epilepsy aged 17 years
and older. TNN
Anaemia pill at schools
The Telegraph, July 16, 2013
Our SPECIAL Special Correspondent New Delhi, July 15: Children in government and
publicfunded schools across India will receive a weekly tablet of iron and folic
acid to reduce anaemia under a programme to be launched this week.
The initiative will cover about 60 million boys and girls enrolled in Classes VI
to XII at government and aided schools, a senior health official said today. It
will also cover 70 million out- of- school girls, aged 10 to 19, under the Integrated
Child Development Scheme.
A nationwide health survey seven years ago had indicated that five in 10 girls and
three in 10 boys aged 15 to 19 have anaemia, which can impair physical growth and
work performance in adolescents. Factors contributing to anaemia include poor intake
of iron- rich food, iron and blood loss because of intestinal worms, and iron loss
during menstruation in girls. Anuradha Gupta, joint secretary in the Union health
ministry, said Rs 135 crore had been set aside for the programme during 2013- 14.

The tablets, containing 100mg iron and 500 micrograms of folic acid each, will be
administered on a fixed day of the week throughout the year. The ministry has asked
the states to earmark Monday as the day. The programme will also seek to provide
a de- worming tablet of albendazole twice a year to these children, once in August
and again in February.
" We could expect children with mild anaemia to become non- anaemic and those with
moderate anaemia to improve to mild anaemia within months," said Umesh Kapil, professor
of human nutrition at AIIMS, New Delhi.
The programme guidelines advise that the children be given these tablets after the
day's main meal to reduce the risk of side effects such as nausea, health officials
said.
Those who report side effects, which can also include stomach pain, will be advised
to take the tablet after dinner, just before sleeping.
Lead poisoning affects 20% city kids Is Your Child Suddenly Slow Or Irritable? Check
For Lead Poisoning
Prithvijit Mitra TNN, July 14, 2013

Kolkata: It’s a silent killer that could be taking a heavy toll on young children.
A study by doctors in Kolkata reveals that at least 20% of the city’s children are
affected by lead poisoning, which is turning out to be a bigger threat than anyone
imagines.
Sixty percent of the Kolkata samples tested positive for lead poisoning. This is
twice the national average. What makes it even scarier is that the symptoms are
too subtle to be noticed. By the time, parents realize something is wrong, the damage
is already done and the child is destined for a lifetime of ailments.
When 10-year-old Rajib Ray started faring poorly in school, his parents thought
he had stopped being attentive in class. His grades steadily went down but his parents
got alarmed only when he failed the final exam. “He was fairly good in studies,
so it was a bit surprising. Then, we found that he couldn’t memorize his lessons
despite trying hard. When we took him to the doctor, we were told that it could
be the fallout of lead poisoning. We got his blood tested and our worst fears came
true. It was probably the lead-based paint on his toys that poisoned him,” said
Ruchira Ray, his mother.

Toys are primarily to blame. Though some of the more reputable firms ensure lead-free
paint and safe plastic, the majority of the toys have toxic paint that is absorbed
even through the baby’s tender skin. A host of other daily-use items may also be
hazardous, like cheap plastic mugs, lead pencils, cheap colours and crayons. Wall
paints can also be very dangerous, warn doctors.
The level of lead in blood should not be more than 10 micorgrams/dL in children
and no more than 20 micrograms/dL for adults. But 150 of the 250 blood samples of
Kolkata kids tested at a Mumbai laboratory showed alarmingly high levels of lead
contamination. Every fifth child in Kolkata is believed to be a victim of lead toxicity.
Children are more susceptible because they have smaller bodies.
The rise in the number of children suffering from irritability, fatigue, weight
loss, memory loss and abdominal pain is directly linked to lead poisoning, says
preventive medicine specialist Debashish Basu, who led a team of five doctors to
conduct the study. “In the long term, it could lead to neurological disorders, bone
damage, muscular weakness and reduced sperm count. It could also lead to high blood
pressure and a decline in mental functioning. By the time these symptoms start hinting
at possible lead poisoning, the damage has been done,” said Basu.
Polluted water and soil, some medicines and cheap cosmetics could also be responsible,
says the study. “In many cases, these products violate the maximum permissible limit
of lead in colour additives. Scratching their surfaces releases lead dust which
easily gets into children. Since lead can’t be ejected by the system, it remains
stored in the body and generates toxicity,” said Basu.
In some cases, children get affected if their parents work in hazardous industries
where lead is used. But in Kolkata, the primary sources of lead toxicity are believed
to paints and pencils used by kids. Paint peeling off the walls can be the most
dangerous as it produced lead dust that easily affects children. “Anaemia and gum
infection among children is often an indicator of lead poisoning. Also, it slows
down cerebral functioning leading to loss of memory and poor academic performance.
These symptoms are significantly common among children in Kolkata,” said critical
care specialist Arindam Kar. Paeditrician Shantanu Ray agreed. “Anaemia is the most
common symptom. But lead poisoning is rarely detected until the child starts having
difficulty in memorizing lessons. Toys and even cooking utensils are often the source
of lead,” said Ray.
The best way to control the health hazards caused due to lead exposure is prevention,
says Sandeep Warghade, in-charge of clinical chemistry at the Metropolis Healthcare
laboratory in Mumbai. “If your child thinks or acts slowly, there could be a reason
to be concerned. Since it is very common among children, consult your physician
if you see the symptoms indicative of lead poisoning. The smaller and growing bodies
of children make them more susceptible to lead,” said Warghade.
Glaxo admits to bribing officials, doctors: China
Saibal Dasgupta TNN, July 12, 2013

Beijing: Senior managers of British pharmaceutical firm GlaxoSmithKline (GSK)’s
local unit have confessed to bribery, “serious” business offences and tax crimes,
the Chinese government has said.
“As a big multinational pharmaceutical company, GSK China in recent years rampantly
bribed some government officials, a number of pharmaceutical industry groups and
funds, hospitals and doctors,” Chinese ministry of public security said in a statement.

It said the firm indulged in bribery to sell products or raise prices. “Money was
distributed via agencies and other channels in the form of direct bribery or sponsorship.’’
The ministry said the company committed tax-related crimes after police investigations.
“The case involves a large number of people, a long period of time, a huge value
and its circumstances are vile,” it added. The statement said some senior managers
have confessed to their wrongdoings in preliminary interrogation without specifying
the suspects’ nationalities.
GSK is one of the largest multinational pharmaceutical companies in China with total
investment of more than $500 million, according to its website.
GSK said it would cooperate with the authorities but said Thursday’s announcement
was the first official communication it has received about the investigation. “Corruption
has no place in our business,” said a company statement. “If evidence of such activity
is provided we would of course act swiftly on it.”
Wockhardt drugs recalled in UK
Times News Network, July 12, 2013
Mumbai: Britain drugs regulator MHRA said on Thursday it has asked Wockhardttorecall16medicines
from pharmacies and wholesalers in the UK after it found deficiencies in manufacturing
procedures at the company’s Waluj plant.
In May, the US Food and Drug Administration had also imposed an “import alert” on
the same plant. Earlier, Wockhardt had said that the FDA’s action potentially affected
around $100 million in revenue on an annualized basis. An “import alert”, effectively
a ban, results in the detention of drugs, without physical examination, from companies
that have not met good manufacturing practices, according to the FDA website.

The MHRA said that the deficiencies found in Wockhardt’s medicines were identified
during a routine inspection in March. It found a low risk of cross-contamination
because of poor cleaning practices and defects in a ventilation system.
The 16 medicines affected by the precautionary recall include those used for the
treatment of infections, high blood pressure, diabetes, epilepsy, depression, Parkinson’s
disease, dementia in Alzheimer’s patients and thyroid conditions.
Gerald Heddell, MHRA’s director of inspection, enforcement and standards, said,
“This is a precautionary recall. People can be reassured that there is no evidence
that medicines made by Wockhardt are defective, so it’s important people continue
to take their medicines as prescribed. However, we have taken this precautionary
action because the medicines have not been manufactured to the right regulatory
standards.”
A Wockhardt spokesperson said the total one-time impact of UK MHRA recall will be
to the tune of 1.5 million pounds (approximately Rs 13.5 crore).
The exports from the Waluj plant constitute less than 5% of total UK sales, and
less than 2% of the overall sales of Wockhardt.
Banned diabetes drug to be back
Pushpa Narayan & Janani Sampath TNN, July 12, 2013
Chennai: Anti-diabetic drug pioglitazone, which was banned by the health ministry
a few days ago, is likely to be back in pharmacies soon. Health ministry sources
told TOI the government decided to revoke the ban on Thursday after a meeting with
12 doctors, a majority of whom argued there was no affordable alternative to the
drug, prescribed for a large number of diabetics in the country.
The ministry will formally revoke the ban on manufacture and sale of the drug within
a week. Till then, it will insist that pharmaceutical companies print a warning
on the cartons. At the meeting, a couple of doctors maintained that the drug carried
the risk of causing bladder cancer, while many others said the ban made diabetes
management ineffective and expensive.

“Most doctors wanted the drug. So we will reintroduce it. But it will be sold with
a warning that high doses can cause bladder cancer. We expect doctors to inform
patients about its risk,” said a senior health department official. “We will send
the report to the health minister and then to the drug technical advisory board,”
he said.
The Chennai-based Madras Diabetes Research Foundation had surveyed 70 diabetologists
across India and found 13 patients had developed bladder cancer after being prescribed
pioglitazone. “None of them had bladder cancer before they were prescribed the drug...
It is possible that even lower dosages increases risk in Indians,” said foundation
director Dr V Mohan.
But many doctors argued that studies linking the drug to bladder cancer were done
in the West, and should not have been banned without scientific evidence about Indians.
Some others said the drug is essential for Indians because it works well on people
with insulin resistance. “These people don’t need insulin. Pioglitazone makes their
natural hormone work like no other drug,” said Dr Vijay Vishwanathan of MV Hospital
for Diabetes.
Dr A Ramachandran, MD of Dr A Ramachandran’s Diabetes Hospital, said it was a knee-jerk
reaction on the government’s part to ban the drug. “All the crosschecking that was
done now should’ve been done before they banned the drug.”
Fresh plea filed against drug price control in SC
Rupali Mukherjee TNN, July 8, 2013

Mumbai: Non-government organisations (NGOs) led by AIDAN (All India Drug Action
Network) have filed a fresh application in the Supreme Court as part of their decade-long
petition that had forced the Centre to bring all 348 essential drugs under price
control. AIDAN, LOCOST, Medico Friend Circle and Jan Swasthya Sahyog together have
filed a fresh intervention application in the Supreme Court, opposing the National
Pharmaceutical Pricing Policy (NPPP 2012) and market-based pricing mechanism to
fix ceiling prices of drugs which are being brought under price control.
According to the fresh intervention application, the simple average formula to determine
the ceiling prices (of drugs) actually increases their prices and legitimizes the
high profit margins already present in the pharma industry. The Drug Price Control
Order (DPCO 2013) and the NPPP 2012 is at best a “leaky bucket”, according to AIDAN.
In addition, the simple average ceiling prices are in many cases higher than the
market leader’s price, it says, adding “nothing could be more absurd”.

Earlier, in November 2011, the organization had moved the Supreme Court, rejecting
the pricing formula in the proposed policy on the grounds that it legitimizes overpricing
and justifies super profits. Through another application earlier, they had asked
the government to bring all combination drugs, patented drugs, life saving drugs
and molecules in the same therapeutic class under price control.
“We want a total clean-up of the therapeutic anarchy and chaos in India. We have
filed our petition the court should hear our objections and then take a call (on
the DPCO 2013)”, says Chinu Srinivasan of LOCOST, co-petitioner in the case. According
to the fresh application filed, in many cases, the DPCO 2013 simple average ceiling
prices are 7.10 times higher than the earlier existing DPCO cost-based prices (see
chart).
Further, citing several examples of the market-based pricing model adopted by the
government in the DPCO 2013, the petition says that profit margins with the new
ceiling prices are over 1000% in many cases. Also, the DPCO 2013 simple average
ceiling price is higher than DPCO 1995 price by over 1000% in five of the 18 drugs
studied.
This shows that the DPCO 2013 ceiling prices are very high compared to the cost
of production. For instance, in the case of atorvastatin 10 mg, which is used to
reduce cholesterol, the market leader price is Rs 97 per 10 tablets, whereas the
price according to costbased pricing under DPCO 1995 norms with 100% margin would
have been Rs 5.60, while the simple average price is around Rs 59.10 per 10 tablets.
Diabetes drug ban to help some cos Pioglitazone Ban To Shift Treatment To Other
Classes Of Medicines
Rupali Mukherjee TNN, The Times Of India Kolkata, Date: Jul 1, 2013, Section:
Times Business

Mumbai: In the wake of a government ban on top-selling diabetes drug, pioglitazone,
the volumes are expected to shift to other classes such as first-generation drugs
(combination of glimepiride and metformin) and newer class of drugs, „gliptins‟,
as well as insulins, even as domestic companies are protesting the ban and examining
all options, including legal recourse.
With the shift to other treatments, companies like Sun Pharma, USV, Glenmark, Sanofi-Aventis
and Lupin will benefit from the move, analysts say. The combination of glimepiride
and metformin is the largest selling product in the anti-diabetic segment, valued
at Rs 823 crore (MAT May 2013, AIOCD AWACS). The oral anti-diabetic market is estimated
at Rs 3,550 crore, while insulins are around Rs 1,100 crore. Pioglitazone, sold
by USV, Sun Pharma, Abbott Healthcare, Micro Labs and Lupin, corners around 20%,
or over Rs 700 crore of the market.

The biggest beneficiary will be the combination of glimepiride and metformin drugs
marketed by USV, Sun Pharma and Lupin. USV clocks nearly Rs 160 crore through this
combination drug, while Sun Pharma mopped up Rs 90 crore and Lupin Rs 70 crore.
The relatively new class of diabetes drugs, gliptins, manufactured by Sun Pharma
and Glenmark, will also benefit from the volume switch while Lupin will benefit
from its brand presence in insulin, an analyst from Nomura said.
In terms of costs for patients prescribed glimepiride plus metformin, there will
be some savings as the combination drug (Rs 4-5) is slightly lower than the combination
of pioglitazone with glimepiride plus metformin (Rs 5.3).
Technically, a shift to gliptins makes sense but there are supply constraints as
the product is patented, as well as expensive, with costs at three to six times
those of pioglitazone combination therapy, he added.
The National Pharmaceutical Pricing Authority has fixed / revised the prices in
respect of more than 150 formulation packs by notification / orders
The National Pharmaceutical Pricing Authority (NPPA) has fixed / revised the prices
in respect of more than 150 formulation packs by notification / orders as per the
DPCO-2013, which are available at
www.nppaindia.nic.in
UK, WHO clean chit to Ranbaxy
Rema Nagarajan, Times of India, June 28, 2013
New Delhi: In yet another twist to the Ranbaxy scandal, the drug regulatory authority
of the UK government has issued a statement clarifying it has found no evidence
that any of Ranbaxy’s products in the UK market “are or have been of unacceptable
quality”. The World Health Organization had issued asimilar statement last month.
Coming within weeks of Ranbaxy agreeing to a $500 million settlement for ‘fraud’
in the US, the two endorsements give the Indian pharma major a much-needed boost.
Equally, they give cause for cheer to generic drug manufacturers in India and other
developing countries, which were fearing being tainted by the Ranbaxy case.
The Medicines and Healthcare products Regulatory Authority (MHRA) of the UK explained
that on hearing that Ranbaxy had pleaded guilty to felony charges related to drugs
made at two facilities in India, it had performed a number of inspections of Ranbaxy
sites along with other international regulators, including the USFDA and the WHO.

While some failures to comply with good manufacturing practice (GMP) were reported,
the inspections did not find any Ranbaxy products in the UK market being either
of unacceptable quality or putting UK patients at risk. MHRA explained how it has
been inspecting Ranbaxy sites since 1995 for GMP compliance and elaborated how,
following USFDA concerns in 2008, it was part of multinational inspection teams
which found that while the company complied in general with European Union GMP,
it needed to demonstrate continued commitment to improving standards of compliance.
Continuing inspections by international teams showed that “the company had made
significant progress”. ‘India is top supplier of APIs’
According to the MHRA, India is the biggest supplier of Active Pharmaceutical Ingredients
(APIs) to the EU and in the last financial year, of the 116 overseas inspections
conducted by MHRA, 47 were performed in India and 52 were performed in the US. "There
is not a noticeable difference in the number of significant findings identified
at UK sites versus those overseas," added the MHRA. In the UK 23% of UK Marketing
Authorisations name an Indian manufacturer and 38% name an Indian API source.
WHO in its statement explained that it has a number of finished pharmaceutical products
(FPPs), mostly HIV/AIDS drugs, manufactured by Ranbaxy on its pre-qualified list
and added that a number of generic medicines prequalified by the WHO Pre-Qualification
Programme (WHO-PQP), including those of Ranbaxy, have also been approved by stringent
regulatory authorities (SRAs). SRAs are those national medicines regulatory authorities
which are members, observers or associates of the International Conference on Harmonization
of Technical Requirements for Registration of Pharmaceuticals for Human Use.
The WHO-PQP statement explained: "In the USA, the term "adulterated" has a specific
legal meaning.”
3 drugs banned over health risks Clampdown On Pioglitazone, Analgin, Deanxit
Rupali Mukherjee, TNN, June 27, 2013
Mumbai: The government has banned three medicines — the widely prescribed anti-diabetes
drug pioglitazone, painkiller analgin and anti-depressant deanxit — over health
risks associated with them.
While it’s believed that pioglitazone can cause heart failure and increases the
risk of bladder cancer, analgin has been discarded worldwide over patient safety.
Deanxit, on the other hand, is a harmful combination long banned even in Denmark,
its country of origin.
This decision follows the government’s strong stand on suspending marketing of all
drugs prohibited for sale in other countries like the US, the UK, EU and Australia.

The ministry of health and family welfare has suspended the manufacture and sale
of all three drugs under Section 26A of the Drugs and Cosmetics Act, 1940 with immediate
effect, through a notification issued on June 18, sources told TOI. While the ministry
had dilly-dallied on withdrawing analgin and deanxit for years despite pressure
from a parliamentary panel, the decision on diabetes drug pioglitazone has taken
the industry by surprise.
The decision to ban pioglitazone and its combinations will hit the Rs 700-crore
market for such drugs and impact a clutch of companies including Abbott, Sun Pharma,
USV, Lupin, Ranbaxy and Wockhardt.
The pioglitazone combination is a bigger market than plain pioglitazone itself,
which has posted doubledigit growth, with over 30 companies marketing the drug.
The topselling brands of posiglitazone include Pioz MF G and Pioz (USV), Gemer P
(Sun Pharma), Tribet (Abbott), Tripride (Micro Labs) and Gluconorm PG (Lupin).
Popular pain-reliever analgin has a relatively small market, with brands like Baralgan
and Novalgin (Sanofi Aventis), as most companies fearing a ban have quit the market,
experts said. The third drug, a combination of Flupenthixol and Melitracen sold
as Deanxit (Lundbeck), Placida (Mankind), Franxit (Intas) and Restfull (Lupin),
is facing a ban because deanxit is prohibited for sale in Denmark, its country of
origin. Also, the combination is not sold in major countries.
CDMU Work in Jharkhand
Background
The state of Jharkhand, India’s twenty-eighth state was carved out of southern Bihar
and came into existence on November 15, 2000. It is bound by Bihar on the north,
West Bengal in the east, Orissa in the south and Chhattisgarh and UP in the west.
It covers an area of 79,714 sq km, with 24 districts, 32,620 villages and a population
of 29.2 million. With 28 percent of the state’s population comprising tribal communities
(compared to the all-India average of 8 percent), Jharkhand was created as a “tribal
state”. Jharkhand is one of the poorest and most backward states in the country
with low per capita income, 54% of the population live below poverty line.
Health sector
The primary health sector in the region appears to be very precarious with a high
level of doctors’ absenteeism: 47 percent of rural respondents reported doctors’
absence in the Public Health Centres (“PHC”). Client satisfaction indicators tell
the same story for rural health. As indicated in the Figure below, the current medical
program of Govt of Jharkhand set up has very low client satisfaction based on basic
medical needs like availability of medicine, children and pregnant women’s treatment
along with doctor’s response. The reasons for poor client satisfaction include:
distance, absenteeism, attitude, inadequate provisioning for maintenance, and low
local-level participation.
Access to medicines – situation in Jharkhand
Healthcare remains a major problem in Jharkhand. Due to its difficult terrain, the
problem gets very severe especially in the isolated and interior tribal villages.
Lack of nutritious food, proper sanitation, potable drinking water, timely health
services and health infrastructures makes life more challenging for the vulnerable
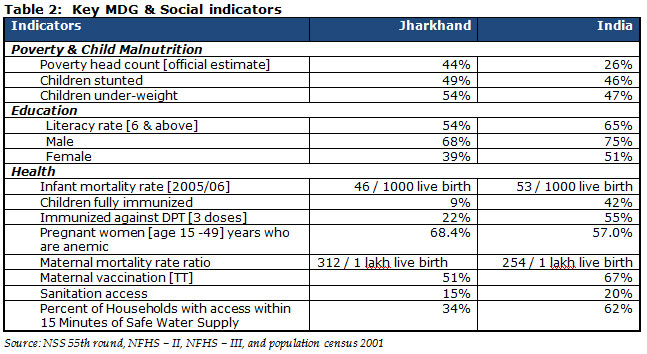
communities. The state’s key social indicators such as literacy, enrolment, infant
mortality and child nutrition, are well below the all-India average. The Table below
narrates the details pertaining to Millennium Development Goals (“MDG”) and social
indicators.

Healthcare delivery needs of the common people cannot be accomplished totally by
the government sector. The government of Jharkhand spent only 4.18 percent of total
government expenditure on health, of which drugs constitute a minimal amount. Majority
of people in Jharkhand has to depend upon private healthcare facilities. Disease,
ill-health, and the financial implications due to health problems are the biggest
risks plaguing most of the people in the region. It has been estimated that in Jharkhand
170,000 [approx] people are moving below poverty level every year. One of the major
reasons is health problem. Within health, the two primary issues are lack of relevant
and appropriate information in healthcare management and lack of access to medicines
at affordable price. An estimate shows that about 3.49% of total house-hold expenditure
goes to healthcare and of which 63.08% goes on buying medicines.
Problem statement
The region of Jharkhand was identified based on the feedback of participants who
attended the follow-up seminar ‘Towards a pharmaceutical policy in India in the
changed perspective’ on September 2, 2010 held at which was organized by CDMU in
collaboration with HAIAP. They suggested that CDMU expand its access to medicines
program in Jharkhand, which is a neighboring state of West Bengal, where CDMU already
has a strong presence and is successfully implementing the program.
CDMU had identified certain issues in the healthcare segment in the Jharkhand region
based on secondary information from organizations that CDMU plans to partner with
to take forward the issue of much importance – the access to essential medicines
by the common people. The problem that the organizations are facing as highlighted
by them is difficulty in respect to medicine access. This was initially revealed
in the feedback and discussions by the participants who participated in the seminar
‘Pharmaceutical policy & access to essential medicines’ organized by CDMU in collaboration
with SIGN, CHABIJ and VHAJ on February 18, 2011 and the workshop entitled ‘Towards
Partnership’ held at Ranchi on March 22, 2011 which was organized by Social Initiatives
for Growth & Networking [SIGN] in collaboration with Catholic Relief Services [CRS].
In Jharkhand, a number of organizations mostly churches contribute significantly
in the sector of healthcare delivery. Medicines constitute an indispensable component
of healthcare and while they prolong life and prevent disease they also have the
potential to hurt and harm. As understood from the various secondary sources, the
clinics which are situated in the remotest areas many a time runs out-of-stock and
has to go for distress purchase. As a result patients when visiting the clinic do
not get full course of medicines and have no choice but to depend on the retail
outlets for their medicines. In such a situation, many a time the patients either
cannot afford to buy the necessary medicines from the retail shop or have to pay
higher price for the medicines. This creates an additional strain on the family
budget of the patient where already out-of-pocket expenditure is more than the family
income. Therefore there is lack of compliance in many cases that result in medicines
resistance e.g in Jharkhand many of the health centres receiving patient having
Chloroquine resistant Vivax Malaria. The final set of issues (not in order of importance
or priority) derived from the feedback of the representatives of the NGOs who participated
in the seminars/workshops are as follows:
- Access to generic medicines
- Even if they get medicines but they have to procure in ‘BRAND’ which is costlier
- Quality of medicines
- Timely and uninterrupted supply of medicine
- Transportation to the health facility
- Lack of training program for the stores personnel
- Getting medicines at affordable price
Besides the above issues, many a times the organizations delivering healthcare are
often managed by people who lack formal training, knowledge and skill to run such
programs. This contributes greatly to irrationality in managing, storing, prescribing
and dispensing of medicines. This has posed a major challenge to implement the rational
drug management program at the organization level. Such organizations usually initiate
their healthcare program but are unable to compete and sustain their programs due
to lack of conceptual clarity on healthcare management, no organized access to medicines,
poor medical stores management, absence of essential medicines list and treatment
guidelines at clinic level.
During the group meeting at diocese level the organizations suggested that CDMU
should take-up following areas immediately.
- To initiate access to medicines program for organizations in Jharkhand
- To prepare a Standard Protocol in respect to treatment of common diseases, stores
management, infection control and use of consumables.
CDMU in collaboration with SIGN and EPN prepare a Standard Treatment guidelines.
TIMES OF INDIA - 3rd Oct 2012
Average life expectancy of Indians rises 4.6 years
Kounteya Sinha TNN
New Delhi: An average Indian lived 4.6 years longer in 2008 compared with a decade
earlier. An average Indian woman lived three years more than her male counterpart
in 2008.
While the life expectancy (LE) at birth for women was 67.7 years, it stood at 64.6
years for men. This was an increase of 2.5 years and 1.8 years, respectively, when
compared to the (LE) in 2002.
According to the latest life expectancy data — to be released by the Registrar General
of India this week after a gap of almost five years — the LE of a rural Indian increased
by 2.2 years between 2002 and 2008. However, the LE of an urban Indian was up by
just 1.2 years over the same period.
Interestingly, an urban female lived 4.9 years longer than a rural female and 7.9
years longer than a rural male. A woman living in rural Kerala had the highest LE
at birth across all categories at 77.2 years. In contrast, LE at birth was lowest
at below 60 years for a rural male in Madhya Pradesh. Rural males also lived longest
in Kerala at 71.2 years, which was 7.7 years longer than the average rural Indian
male.

Kolkata in network of age-friendly cities
Kolkata has taken the lead to become southeast Asia’s first elderly-friendly city.
On Monday, the International Day of Older Persons, it joined mega cities like New
York, La Plata (Argentina), Canberra and Geneva to become part of the WHO’s Global
Network of Age-friendly Cities and Communities. P 5 Urban women in Kerala live the
longest Kerala’s rural female LE of 77.2 years was also 10.7 years more than the
average rural female LE in the country. Interestingly, women seem to have a longer
life span than their male counterparts in most of the major states.
In West Bengal and Rajasthan, for example, women lived 3.6 years longer than their
male counterparts in 2008. In Tamil Nadu, women lived 3.8 years longer, while in
Punjab it was 4.2 years, Maharashtra (4 years), Haryana (2.5 years), Gujarat (4.1
years), Himachal Pradesh (4.7 years), Karnataka (4.8 years) and Kerala (5.4 years).
When it comes to urban males, Himachal Pradesh took the top spot with an average
man living till 72.6 years at 4.6 years longer than an average urban Indian male.
An urban female was living longest in Kerala — 76.4 years, which was five years
longer than an average urban Indian female. Deputy registrar general Bhaskar Mishra
said, “LE of an average Indian is improving by the year. What is most interesting
is the widening gap between LE of an Indian male and a female which is a trend similar
to developed countries. This may be because of the rapid bridging of the gap in
child mortality between males and females. The previous data on LE came out in 2007.”
So what are the main reasons for Indians living longer now than a decade ago? Experts
say that the three big reasons for LE to have increased are better food supply and
nutrition, healthier lifestyle and better hygiene. “More people are interested in
the nutritional content of the food they eat and plan their diet accordingly. People
are consciously making better lifestyle choices that reduce chances or delay the
risk of developing diseases. People wash their hands more often that reduces infection
rate,” a health ministry official said.
‘Irrational use of medicines rampant’
TIMES NEWS NETWORK
Kolkata: A prescription audit carried out by CUTS, a consumer movement society,
revealed a shocking picture of irrational use of medicines by the city doctors.
About 98.03% prescriptions were found to be irrational, while only 1.96% bore the
testimony of rational use of drugs.
The survey revealed that most irrationally prescribed drugs were antibiotics, NSAIDs,
PPI (proton pump inhibitors), H2 blockers, vitamins, antipsychotics and antihistaminic
(allergic). The study, carried out in private hospitals and nursing homes, revealed
that only a few hospitals had mechanisms for monitoring the compliance to rational
use of drugs (RUD).

Such irrational use of drugs has long-lasting impact on human body, making it immune
to the drug, particularly antibiotics, besides the side effects, said Prithviraj
Nath of CUTS, who conducted the survey.
The study also laid bare the nexus between the doctors and pharmaceutical companies.
Twenty out of 50 pharmaceutical firms were found to have sponsored events, workshops
for doctors. The expenditure on gifts, seminars is added to the price of medicines.
The study revealed that doctors often prescribe expensive medicines, in spite of
availability of cheaper versions in the market.
Reacting to the survey, the minister of state for health Chandrima Bhattacharya,
said, “The right to health is a fundamental right of every citizen of this country
and no entity can infringe upon that right.” She added that from October 2, fifteen
fair-price medicine shops will be operating to facilitate the sale of low-priced
generic medicines to common people. “Eventually, there will be 35 such shops in
the state,” she had said at a seminar jointly organized by CUTS and the department
of health and family welfare recently.
Sanghamitra Ghosh, managing director, West Bengal Medical Services Corporation Ltd,
expressed serious concern over pricing of medicines. “We have to be careful that
high prices of medicines and services should not lead to oppression and exploitation,
especially of the ordinary consumers.” She also stressed on the low awareness levels
of most stakeholders and said that civil society should play an important role in
improving the situation.
HEALTH SCARE
About 98.03% prescriptions were found to be irrational, while only 1.96% bore the
testimony of rational use of drugs The survey by CUTS, a consumer movement society,
revealed that most irrationally prescribed drugs were antibiotics, NSAIDs, PPI (proton
pump inhibitors), H2 blockers, vitamins, antipsychotics and antihistaminic (allergic)
The study also laid bare the nexus between the doctors and pharmaceutical companies.
Twenty out of 50 pharmaceutical companies were found to have sponsored events, workshops
for doctors From October 2, fifteen fair-price medicine shops will be operating
in the state to facilitate the sale of low-priced generic medicines to common people
Poor access to drugs adds to cancer pain India, Other Nations Fail To Provide All
7 Meds
Kounteya Sinha TNN
New Delhi: Untreated cancer pain has become a global pandemic. Pain can affect as
many as 64% of patients with metastatic, advanced or terminal phase disease, 59%
of patients on anti-cancer treatment, and 33% of patients after curative treatment.
However, a global survey — conducted between December 2010 and July 2012, that came
up with 156 reports submitted by experts in 76 countries and 19 Indian states —
has revealed that several countries, including India, have failed to ensure adequate
access to pain-relieving drugs.
The new data, released during the Congress of the European Society for Medical Oncology
on Saturday, paints a dismal picture of unnecessary pain on a global scale.

The researchers found that very few countries provided all seven of the opioid medications
that are considered essential for the relief of cancer pain. Those essential medications
include codeine, immediate and slow release oral morphine, oral oxycodone and transdermal
fentanyl.
In many countries, fewer than three of the seven medications are available. And
the medications that are available are either unsubsidized or marginally subsidized
by government, and the availability is often limited.

In addition, many countries have highly restrictive regulations that limit entitlement
of cancer patients to receive prescriptions, limit prescriber privileges, impose
restrictive limits on duration of prescription, restrict dispensing, and increase
bureaucratic burden of the prescribing and dispensing process.
The good news, however, is that morphine availability may soon be relaxed for medical
use to help reduce acute and chronic pain suffered by terminally ill cancer and
HIV patients. Even better, the increased availability will not just be for patients
admitted in hospitals but also for those under home-based care.
Commenting on the study, Dr Carla Ripamonti from the National Cancer Institute of
Milan said: “Despite published guidelines and educational programmes on the assessment
and treatment of cancer-related pain, unrelieved pain continues to be a substantial
worldwide public health concern in patients with solid cancers and hematological
malignancies.”
WHO STUDY
50-plus Indians at risk from chronic diseases
Kounteya Sinha TNN
New Delhi:This should serve as a wake-up call for India’s 50-plus club, who face
a serious risk from chronic diseases.
A prevalence of risk factors study by the World Health Organization (WHO) conducted
this year among males and females aged 50 or older across six countries, including
India, has some worrying findings for Indians.
According to the Study on Global Ageing and Adult Health (SAGE), 87.9% men and 93.5%
women in this age group have insufficient nutrition intake, while 24% men and 26%
women have low physical activity. Around one in four men and an equal number of
women suffer from high blood pressure. Nearly 63% men and 30% women are daily smokers.
Almost three in four men aged 50 and above and over four in five women have high
risk waist-hip ratio or abdominal obesity, which greatly increases cardiovascular
disease risk.

Nearly 1.3% males in above 50 are obese. The case is worse for Indian women since
3% of them are obese, according to United Nations Population Fund’s (UNFPA) report
on ‘Ageing in the 21st century’, to be released on Monday.
“Risk factors for chronic diseases (such as smoking) vary by country. For example,
63% of men over 50 in India smoke, compared with only 11% in Ghana. In China, 51%
of women over 50 have high blood pressure, compared with 27% in India. The biggest
underlying risk factor for chronic disease in older people is high blood pressure,
which can explain 12 to 19% of the total burden of disease in developing countries,”
says the UN report.
India has around 90 million elderly and the figure is expected to increase to 315
million, constituting 20% of the total population, by 2050.
What should further wake up the Indian 50-plus club is a separate Indian study that
confirms a steep out-of- pocket expenditure to pay health bills. It has been conducted
jointly by UNFPA, Institute for Social and Economic Change (Bangalore), the Institute
for Economic Growth (New Delhi) and the Tata Institute of Social Sciences (Mumbai)
in seven states having a higher elderly population — Kerala, Tamil Nadu, Maharashtra,
Orissa, West Bengal, Punjab and Himachal Pradesh.
Those who were hospitalized (9%) in India spent 10 days in hospital on an average
per episode and spent over Rs 8,800 on consultation, medicines and diagnostics.
In the case of out-patient treatment, the average expenditure was about Rs 1,230.
An elderly person spent Rs 500 every month towards medicines. And only 24% of the
elderly went for general health check-ups spending about Rs 600 each time.